Learning Objectives
- Use Avogadro's number to convert to moles and vice versa given the number of particles of an element.
- Use the molar mass to convert to grams and vice versa given the number of moles of an element.
There is no formula to convert grams into atoms directly. First you must convert grams into moles and them convert moles into atoms. Obtain the molar mass of the element/compound from the periodic table. Remember, the number of atoms in a mole is Avagadro's number, 6.022 x 10^23. Say you have 21 grams of imidazole. How many atoms of nitrogen are there in that 21 grams of imidazole? You will need the following conversion factors: molar mass (68.077 g/mol, the number of molecules per mol, and the number of nitrogen atoms in one molecule (2). Number of Atoms (n) and Number Density (N) The number of atoms or molecules (n) in a mass (m) of a pure material having atomic or molecular weight (M) is easily computed from the following equation using Avogadro's number (NA = 6.022×10 23 atoms or molecules per gram-mole): M mN n A (1).
When objects are very small, it is often inconvenient, inefficient, or even impossible to deal with the objects one at a time. For these reasons, we often deal with very small objects in groups, and have even invented names for various numbers of objects. The most common of these is 'dozen' which refers to 12 objects. We frequently buy objects in groups of 12, like doughnuts or pencils. Even smaller objects such as straight pins or staples are usually sold in boxes of 144, or a dozen dozen. A group of 144 is called a 'gross'.

This problem of dealing with things that are too small to operate with as single items also occurs in chemistry. Atoms and molecules are too small to see, let alone to count or measure. Chemists needed to select a group of atoms or molecules that would be convenient to operate with.
Avogadro's Number and Mole
In chemistry, it is impossible to deal with a single atom or molecule because we can't see them, count them, or weigh them. Chemists have selected a number of particles with which to work that is convenient. Since molecules are extremely small, you may suspect this number is going to be very large, and you are right. The number of particles in this group is (6.02 times 10^{23}) particles and the name of this group is the mole (the abbreviation for mole is (text{mol})). One mole of any object is (6.02 times 10^{23}) of those objects. There is a particular reason that this number was chosen and this reason will become clear as we proceed.
When chemists are carrying out chemical reactions, it is important that the relationship between the numbers of particles of each reactant is known. Any readily measurable mass of an element or compound contains an extraordinarily large number of atoms, molecules, or ions, so an extremely large numerical unit is needed to count them. The mole is used for this purpose.
The mole (symbol: mol) is the base unit of amount of substance ('number of substance') in the International System of Units or System International (SI), defined as exactly 6.02214076×1023 particles, e.g., atoms, molecules, ions or electrons. The current definition was adopted in November 2018, revising its old definition based on the number of atoms in 12 grams of carbon-12 (12C) (the isotope of carbon with relative atomic mass 12 Daltons, by definition). For most purposes, 6.022 × 1023 provides an adequate number of significant figures. Just as 1 mole of atoms contains 6.022 × 1023 atoms, 1 mole of eggs contains 6.022 × 1023 eggs. This number is called Avogadro’s number, after the 19th-century Italian scientist who first proposed a relationship between the volumes of gases and the numbers of particles they contain.
Grams To Atoms Formula
It is not obvious why eggs come in dozens rather than 10s or 14s, or why a ream of paper contains 500 sheets rather than 400 or 600. The definition of a mole—that is, the decision to base it on 12 g of carbon-12—is also arbitrary. The important point is that 1 mole of carbon—or of anything else, whether atoms, compact discs, or houses—always has the same number of objects: 6.022 × 1023.
Grams To Atoms Calcium
Converting Between Number of Atoms to Moles and Vice Versa
We can use Avogadro's number as a conversion factor, or ratio, in dimensional analysis problems. If we are given the number of atoms of an element X, we can convert it into moles by using the relationship
[text{1 mol X} = 6.022 times 10^{23} text{ X atoms}.]

Example (PageIndex{1}): Moles of Carbon
The element carbon exists in two primary forms: graphite and diamond. How many moles of carbon atoms is (4.72 times 10^{24}) atoms of carbon?
Solution
| Steps for Problem Solving | The element carbon exists in two primary forms: graphite and diamond. How many moles of carbon atoms is (4.72 times 10^{24}) atoms of carbon? |
|---|---|
| Identify the 'given' information and what the problem is asking you to 'find.' | Given: (4.72 times 10^{24}) C atoms Find: mol C |
| List other known quantities. | (1, mol = 6.022 times 10^{23}) C atoms |
Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | [4.72 times 10^{24} : cancel{text{C} : ce{atoms}} times frac{1 : text{mol} : ce{C}}{6.02 times 10^{23} : cancel{text{C} : ce{atoms}}} = 7.84 : text{mol} : ce{C} nonumber] |
| Think about your result. | The given number of carbon atoms was greater than Avogadro's number, so the number of moles of (ce{C}) atoms is greater than 1 mole. Since Avogadro's number is a measured quantity with three significant figures, the result of the calculation is rounded to three significant figures. |
Molar Mass
Molar mass is defined as the mass of one mole of representative particles of a substance. By looking at a periodic table, we can conclude that the molar mass of the element lithium is 6.94g, the molar mass of zinc is 65.38g, and the molar mass of gold is 196.97g. Each of these quantities contains (6.022 times 10^{23}) atoms of that particular element. The units for molar mass are grams per mole or g/mol. (1.00 : text{mol}) of carbon-12 atoms has a mass of (12.0 : text{g}) and contains (6.022 times 10^{23}) atoms. 1.00 mole of any element has a mass numerically equal to its atomic mass in grams and contains (6.022 times 10^{23}) particles. The mass, in grams, of 1 mole of particles of a substance is now called the molar mass (mass of 1.00 mole).
Converting Grams to Moles of an Element and Vice Versa
We can also convert back and forth between grams of an element and moles. The conversion factor for this is the molar mass of the substance. The molar mass is the ratio giving the number of grams for each one mole of the substance. This ratio is easily found by referring to the atomic mass of the element using the periodic table. This ratio has units of grams per mole or (text{g/mol}).
Conversions like this are possible for any substance, as long as the proper atomic mass, formula mass, or molar mass is known (or can be determined) and expressed in grams per mole. Figure 6.4.1 illustrates what conversion factor is needed and two examples are given below.
Example (PageIndex{2}): Chromium
Chromium metal is used for decorative electroplating of car bumpers and other surfaces. Find the mass of 0.560 moles of chromium.
Solution
| Steps for Problem Solving | Chromium metal is used for decorative electroplating of car bumpers and other surfaces. Find the mass of 0.560 moles of chromium. |
|---|---|
| Identify the 'given' information and what the problem is asking you to 'find.' | Given: 0.560 mol Cr Find: g Cr |
| List other known quantities. | 1 mol Cr = 52.00g Cr |
Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | [0.560 : cancel{text{mol} : ce{Cr}} times frac{52.00 : text{g} : ce{Cr}}{1 : cancel{text{mol} : ce{Cr}}} = 29.1 : text{g} : ce{Cr} nonumber] |
| Think about your result. | Since the desired amount was slightly more than one half of a mole, the mass should be slightly more than one half of the molar mass. The answer has three significant figures because of the (0.560 : text{mol}) |
Example (PageIndex{3}): Silicon
How many moles are in 107.6g of Si?
Solution
| Steps for Problem Solving | How many moles are in 107.6g of Si. |
|---|---|
| Identify the 'given' information and what the problem is asking you to 'find.' | Given: 107.6g Si Find: mol Si |
| List other known quantities. | 1 mol Si = 28.09g Si |
Prepare a concept map and use the proper conversion factor. | |
| Cancel units and calculate. | [107.6 : cancel{text{g} : ce{Si}} times frac{1 : text{mol} : ce{Si}}{28.09 : cancel{text{g} : ce{Si}}} = 3.83 : text{mol} : ce{Si}] |
| Think about your result. | Since 1 mol of Si is 28.09g, 107.6 should be about 4 moles. |
Exercise (PageIndex{1})
- How many moles are present in 100.0 g of Al?
- What is the mass of 0.552 mol of Ag metal?
- Answer a:
- 3.706 mol Al
- Answer b:
- 59.5 g Ag
Summary
- A mole is defined as exactly 6.02214076×1023 particles, e.g., atoms, molecules, ions or electrons.
- There are (6.02214076 times 10^{23}) particles in 1.00 mole. This number is called Avogadro's number.
- The molar mass of an element can be found by referring to the atomic mass on a periodic table with units of g/mol.
- Using dimensional analysis, it is possible to convert between grams, moles, and the number of atoms or molecules.
Further Reading/Supplemental Links
- learner.org/resources/series61.html - The learner.org website allows users to view streaming videos of the Annenberg series of chemistry videos. You are required to register before you can watch the videos, but there is no charge. The website has one video that relates to this lesson called The Mole.
- Using Avogadro's law, the mass of a substance can be related to the number of particles contained in that mass. The Mole: (www.learner.org/vod/vod_window.html?pid=803)
- Vision Learning tutorial: The Mole http://visionlearning.com/library/mo...p?mid-53&1=&c3=
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
Marisa Alviar-Agnew (Sacramento City College)
Henry Agnew (UC Davis)
- Wikipedia
Using the grid & bridge concepts(Std 3inCh 8)
bridge 1bridge 2bridge 3
milligrams<--->grams<--->moles<--->atoms or molecules
Bridges are fractions that equal 1.They allow us to change one unit of measure to another, like crossing a bridge over a river from one side to the other.Notice that each bridge can go in two directions, just like a real bridge over a river.For a bridge to work, the top and bottom of the fraction must equal each other .That is why the value of all bridges = 1. |
The following equivalent equations will be made into bridges (fractions that = 1) to do go back and forth from one unit of measure to another:
bridge type 1 (3 kinds)
1000 milligrams = 1 gram

1000 grams = 1 kg
1.0 x 106mg (microgram) = 1 gram
bridge type 2
1 molar mass in grams (or gram molecular wt if we are using elements) = 1 mole
bridge type 3
6.022 x 1023atoms (or molecules)=1 mole(remember Avogadro !!! )
Your job is to create fractions that will take you the way you need to go.The example will use both the bridge concept and conversion grids.Here’s the example:
Example 1
You are given 850 mg ofCa and asked to find out how many atoms that represents.Each bridge is made as follows:
Solution – Ex 1
bridge 11000 mg = 1 g
bridge 240.1 g Ca=1 mole Ca
bridge 31 mole=6.02 x 1023 atoms
We make each fraction from the bridge that takes us from one unit of measure to another.The side of the fraction that goes on top, is the one with unit of measure we are changing to.Here is how it works:
starting fraction:850 mg/1 (the number one)ending fraction:atoms/1
bridge 1bridge 2bridge 3
850 mg Ca | 1 g | 1 mol Ca | 6.02 x 1023atoms |
1 | 1000 mg | 40.1 g Ca | 1 mol Ca |
Now let’s see what the grid looks like when we cancel units:
850 | 1 | 1 mol | 6.02 x 1023Ca atoms |
1 | 1000 | 40.1 | 1 mol |
The final fraction looks like this:
5,117 x 1023Ca atoms |
40,100 |
Now all we have to do is divide the two numbers at the end:
0.128 x 1023 Ca atoms
For correct scientific notation we must move the decimal one place to the right.When we do that we must adjust the exponent of 10 to be one number less, or we change the value of the number.
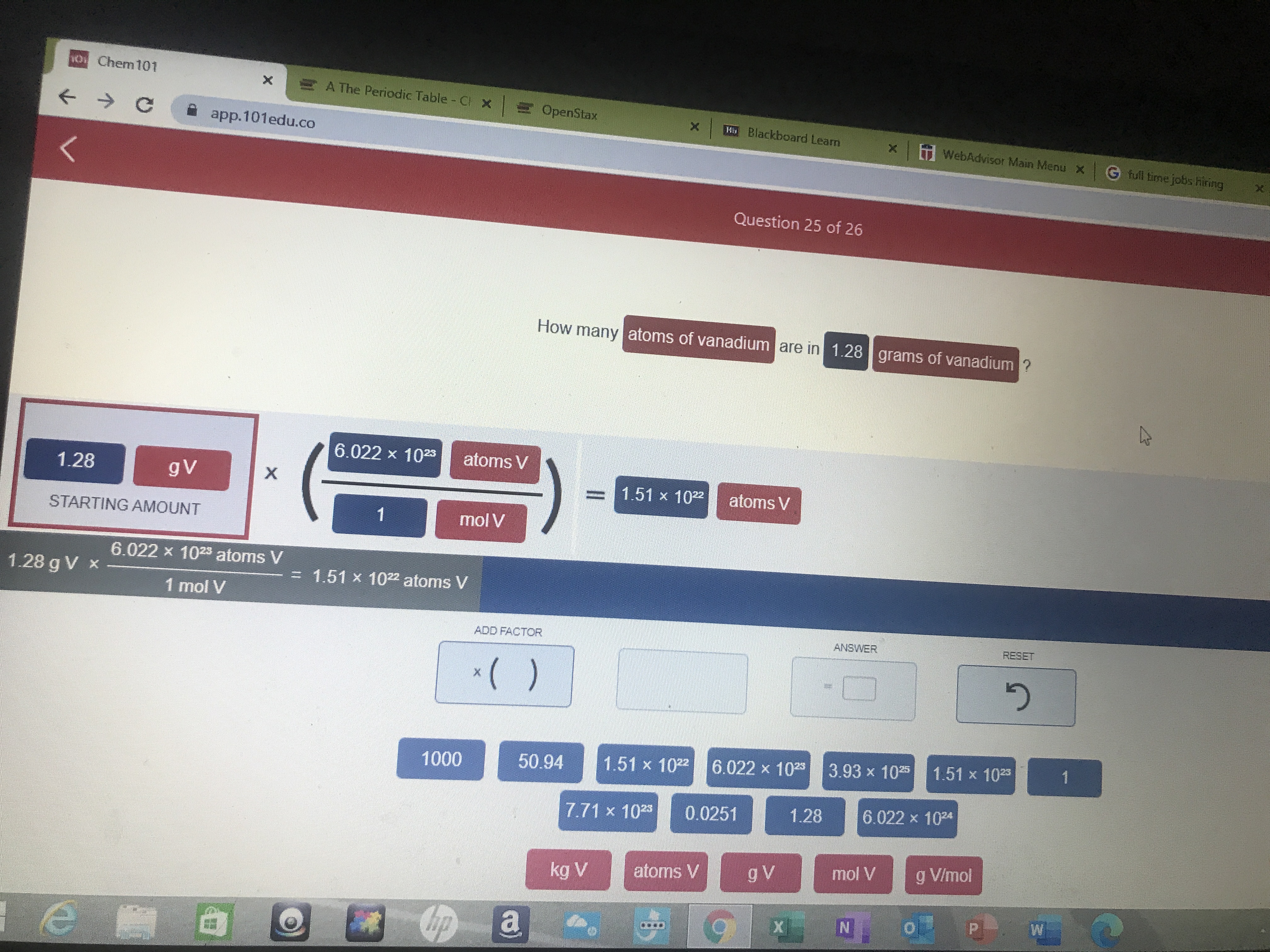
FINAL ANSWER =1.28 x 1022Ca atoms
Example 2
You are given4.35 x 1019 atoms of Mg.You are supposed to find how many grams that represents.
Solution
First let’s see what the grid looks like:
bridge 1bridge 2bridge 3
4.35 x 1019 atoms Mg | 1 mole | 24.3 g Mg |
1 | 6.20 x 1023 atoms | 1 mole Mg |
Now let’s cancel units and see what is left:
4.35 x 1019 | 1 | 24.3 g Mg |
1 | 6.02 x 1023 | 1 |
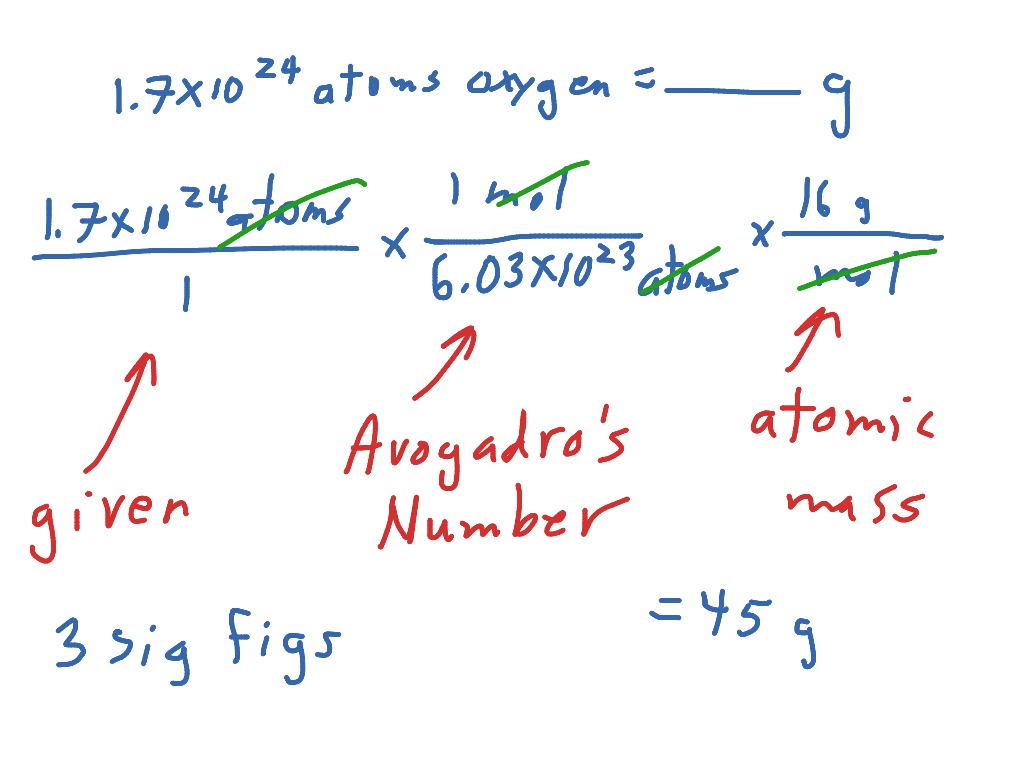
This is equal to:
105.7 x 1019 g Mg |
6.02 x 1023 |
As in Example 1, we now divide the numbers.This time though we do the “normal” numbers separately from the 10’s with the exponents:
105.7 / 6.02 =17.61019/1023=10-4
Now we “reassemble” the numbers to read
17.6 x 10-4
This time to change to scientific notation we must move the decimal to the left one place.In order to keep the same number we must adjust the exponent of 10 to be 3.
FINAL ANSWER =1.76 x 10-3
If this didn’t make sense to you, go back over it slowly a few times and let it “sink in”.
