The elements of the periodic table sorted by atomic mass
.chlorine has two naturally occurring isotopes. The mass of chlorine-35 is 34.696 amu and the mass of chlorine-37 is 36.966 amu. Using the average mass from the periodic table (average atomic mass of chlorine is 35.453), find the abundance of each isotope. (remember that the sum of the two abundances must be 100). In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37. To calculate the relative atomic mass, A r, of chlorine. The atomic mass of chlorine is 35.5. It has two isotopes of atomic mass 35 and 37. What is the percentage of a heavier isotope in the sample? If the percentage of heavy Chlorine atoms is a then the percentage of lighter ones is 100-a. The average atomic mass of chlorine atom can be calculated by using weighted averages. 75.8% of chlorine has the atomic mass of 35 g/mole and 24.2% has an atomic mass of 37 g/mole. The average atomic mass of the given sample of chlorine is 35.46 u.
click on any element's name for further information on chemical properties, environmental data or health effects.
Chlorine Atomic Mass And Atomic Number
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic Mass | Name chemical element | Symbol | Atomic number |
| - Name alphabetically | 1.0079 | Hydrogen | H | 1 |
| - Atomic number | 4.0026 | Helium | He | 2 |
| - Symbol | 6.941 | Lithium | Li | 3 |
| - Atomic Mass | 9.0122 | Beryllium | Be | 4 |
| - Electronegativity | 10.811 | Boron | B | 5 |
| - Density | 12.0107 | Carbon | C | 6 |
| - Melting point | 14.0067 | Nitrogen | N | 7 |
| - Boiling point | 15.9994 | Oxygen | O | 8 |
| - Vanderwaals radius | 18.9984 | Fluorine | F | 9 |
| - Year of discovery | 20.1797 | Neon | Ne | 10 |
| - Inventor surname | 22.9897 | Sodium | Na | 11 |
| - Elements in earthcrust | 24.305 | Magnesium | Mg | 12 |
| - Elements in human body | 26.9815 | Aluminum | Al | 13 |
| - Covalenz radius | 28.0855 | Silicon | Si | 14 |
| - Ionization energy | 30.9738 | Phosphorus | P | 15 |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). The lightest chemical element is Hydrogen and the heaviest is Hassium. The unity for atomic mass is gram per mol. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 32.065 | Sulfur | S | 16 |
| 35.453 | Chlorine | Cl | 17 | |
| 39.0983 | Potassium | K | 19 | |
| 39.948 | Argon | Ar | 18 | |
| 40.078 | Calcium | Ca | 20 | |
| 44.9559 | Scandium | Sc | 21 | |
| 47.867 | Titanium | Ti | 22 | |
| 50.9415 | Vanadium | V | 23 | |
| 51.9961 | Chromium | Cr | 24 | |
| 54.938 | Manganese | Mn | 25 | |
| 55.845 | Iron | Fe | 26 | |
| 58.6934 | Nickel | Ni | 28 | |
| 58.9332 | Cobalt | Co | 27 | |
| 63.546 | Copper | Cu | 29 | |
| 65.39 | Zinc | Zn | 30 | |
| 69.723 | Gallium | Ga | 31 | |
| 72.64 | Germanium | Ge | 32 | |
| 74.9216 | Arsenic | As | 33 | |
| 78.96 | Selenium | Se | 34 | |
| 79.904 | Bromine | Br | 35 | |
| 83.8 | Krypton | Kr | 36 | |
| 85.4678 | Rubidium | Rb | 37 | |
| 87.62 | Strontium | Sr | 38 | |
| 88.9059 | Yttrium | Y | 39 | |
| 91.224 | Zirconium | Zr | 40 | |
| 92.9064 | Niobium | Nb | 41 | |
| 95.94 | Molybdenum | Mo | 42 | |
| 98 | Technetium | Tc | 43 | |
| 101.07 | Ruthenium | Ru | 44 | |
| 102.9055 | Rhodium | Rh | 45 | |
| 106.42 | Palladium | Pd | 46 | |
| 107.8682 | Silver | Ag | 47 | |
| 112.411 | Cadmium | Cd | 48 | |
| 114.818 | Indium | In | 49 | |
| 118.71 | Tin | Sn | 50 | |
| 121.76 | Antimony | Sb | 51 | |
| 126.9045 | Iodine | I | 53 | |
| 127.6 | Tellurium | Te | 52 | |
| 131.293 | Xenon | Xe | 54 | |
| 132.9055 | Cesium | Cs | 55 | |
| 137.327 | Barium | Ba | 56 | |
| 138.9055 | Lanthanum | La | 57 | |
| 140.116 | Cerium | Ce | 58 | |
| 140.9077 | Praseodymium | Pr | 59 | |
| 144.24 | Neodymium | Nd | 60 | |
| 145 | Promethium | Pm | 61 | |
| 150.36 | Samarium | Sm | 62 | |
| 151.964 | Europium | Eu | 63 | |
| 157.25 | Gadolinium | Gd | 64 | |
| 158.9253 | Terbium | Tb | 65 | |
| 162.5 | Dysprosium | Dy | 66 | |
| 164.9303 | Holmium | Ho | 67 | |
| 167.259 | Erbium | Er | 68 | |
| 168.9342 | Thulium | Tm | 69 | |
| 173.04 | Ytterbium | Yb | 70 | |
| 174.967 | Lutetium | Lu | 71 | |
| 178.49 | Hafnium | Hf | 72 | |
| 180.9479 | Tantalum | Ta | 73 | |
| 183.84 | Tungsten | W | 74 | |
| 186.207 | Rhenium | Re | 75 | |
| 190.23 | Osmium | Os | 76 | |
| 192.217 | Iridium | Ir | 77 | |
| 195.078 | Platinum | Pt | 78 | |
| 196.9665 | Gold | Au | 79 | |
| 200.59 | Mercury | Hg | 80 | |
| 204.3833 | Thallium | Tl | 81 | |
| 207.2 | Lead | Pb | 82 | |
| 208.9804 | Bismuth | Bi | 83 | |
| 209 | Polonium | Po | 84 | |
| 210 | Astatine | At | 85 | |
| 222 | Radon | Rn | 86 | |
| 223 | Francium | Fr | 87 | |
| 226 | Radium | Ra | 88 | |
| 227 | Actinium | Ac | 89 | |
| 231.0359 | Protactinium | Pa | 91 | |
| 232.0381 | Thorium | Th | 90 | |
| 237 | Neptunium | Np | 93 | |
| 238.0289 | Uranium | U | 92 | |
| 243 | Americium | Am | 95 | |
| 244 | Plutonium | Pu | 94 | |
| 247 | Curium | Cm | 96 | |
| 247 | Berkelium | Bk | 97 | |
| 251 | Californium | Cf | 98 | |
| 252 | Einsteinium | Es | 99 | |
| 257 | Fermium | Fm | 100 | |
| 258 | Mendelevium | Md | 101 | |
| 259 | Nobelium | No | 102 | |
| 261 | Rutherfordium | Rf | 104 | |
| 262 | Lawrencium | Lr | 103 | |
| 262 | Dubnium | Db | 105 | |
| 264 | Bohrium | Bh | 107 | |
| 266 | Seaborgium | Sg | 106 | |
| 268 | Meitnerium | Mt | 109 | |
| 272 | Roentgenium | Rg | 111 | |
| 277 | Hassium | Hs | 108 | |
| Darmstadtium | Ds | 110 | ||
| Copernicium | Cn | 112 | ||
| Nihonium | Nh | 113 | ||
| Flerovium | Fl | 114 | ||
| Moscovium | Mc | 115 | ||
| Livermorium | Lv | 116 | ||
| Tennessine | Ts | 117 | ||
| Oganesson | Og | 118 |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Lenntech (European Head Office)
Distributieweg 3
2645 EG Delfgauw
The Netherlands
Phone: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Lenntech USA LLC (Americas)
5975 Sunset Drive
South Miami, FL 33143
USA
Phone: +1 877 453 8095
e-mail: info@lenntech.com
Lenntech DMCC (Middle East)
Level 5 - OFFICE #8-One JLT Tower
Jumeirah Lake Towers
Dubai - U.A.E.
Phone: +971 4 429 5853
e-mail: info@lenntech.com
Copyright © 1998-2021 Lenntech B.V. All rights reserved
Imagine that you have a pile of rocks to move, and need to decide what equipment to rent so that you can move them. If the rocks are fairly small, you can get a shovel to pick them up. Larger rocks could be moved by hand, but big boulders will need some sort of mechanical scoop. The amount of each kind of rock will also determine how much time you will need to get the job done. Knowing the relative amounts of large, medium, and small rocks can be very useful in deciding how to approach the job.
Percent Natural Abundance
Most elements occur naturally as a mixture of two or more isotopes. The table below shows the naturally occuring isotopes of several elements, along with the percent natural abundance of each.
| Element | Isotope (Symbol) | Percent Natural Abundance | Atomic Mass (left( text{amu} right)) | Average Atomic Mass (left( text{amu} right)) |
|---|---|---|---|---|
| Hydrogen | (ce{_1^1H}) | 99.985 | 1.0078 | 1.0079 |
| (ce{_1^2H}) | 0.015 | 2.0141 | ||
| (ce{_1^3H}) | negligible | 3.0160 | ||
| Carbon | (ce{_6^{12}C}) | 98.89 | 12.000 | 12.011 |
| (ce{_6^{13}C}) | 1.11 | 13.003 | ||
| (ce{_6^{14}C}) | trace | 14.003 | ||
| Oxygen | (ce{_8^{16}O}) | 99.759 | 15.995 | 15.999 |
| (ce{_8^{17}O}) | 0.037 | 16.995 | ||
| (ce{_8^{18}O}) | 0.204 | 17.999 | ||
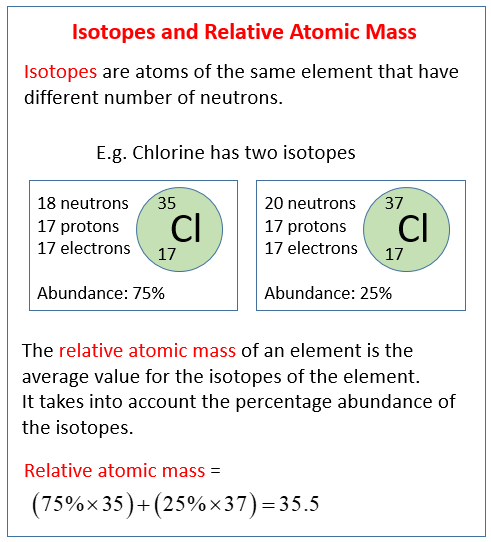
| Chlorine | (ce{_{17}^{35}Cl}) | 75.77 | 34.969 | 35.453 |
| (ce{_{17}^{37}Cl}) | 24.23 | 36.966 | ||
| Copper | (ce{_{29}^{63}Cu}) | 69.17 | 62.930 | 63.546 |
| (ce{_{29}^{65}Cu}) | 30.83 | 64.928 |
For some elements, one particular isotope predominates greatly over the other isotopes. Naturally occurring hydrogen is nearly all hydrogen-1 and naturally occurring oxygen is nearly all oxygen-16. For many other elements, however, more than one isotope may exist in more substantial quantities. Chlorine (atomic number 17) is a yellowish-green toxic gas. About three quarters of all chlorine atoms have 18 neutrons, giving those atoms a mass number of 35. About one quarter of all chlorine atoms have 20 neutrons, giving those atoms a mass number of 37. Were you to simply calculate the arithmetic average of the precise atomic masses, you would get 36.
[frac{left( 34.969 + 36.966 right)}{2} = 35.968 : text{amu}]
Clearly the actual average atomic mass from the last column of the table is significantly lower. Why? We need to take into account the percent natural abundance of each isotope in order to calculate what is called the weighted average. The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element. The sample problem below demonstrates how to calculate the atomic mass of chlorine.
Example (PageIndex{1})
Use the atomic masses of each of the two isotopes of chlorine along with the respective percent natural abundance to calculate the average atomic mass of chlorine.
Solution
Step 1: List the known and unknown quantities and plan the problem.
Known
- Chlorine-35: atomic mass (= 34.969 : text{amu}) and percent abundance (= 75.77%)
- Chlorine-37: atomic mass (= 36.966 : text{amu}) and percent abundance (= 24.23%)
Chlorine Periodic Table
Unknown
- Average atomic mass of chlorine
Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
Step 2: Calculate.
[begin{array}{ll} text{chlorine-35} & 0.7577 times 34.969 = 26.50 : text{amu} text{chlorine-37} & 0.2423 times 36.966 = 8.957 : text{amu} text{average atomic mass} & 26.50 + 8.957 = 35.45 : text{amu} end{array}]
Note: Applying significant figure rules results in the (35.45 : text{amu}) result without excessive rounding error. In one step:
[left( 0.7577 times 34.969 right) + left(0.2423 times 36.966 right) = 35.45 : text{amu}]
Step 3: Think about your result.
The calculated average atomic mass is closer to 35 than to 37 because a greater percentage of naturally occurring chlorine atoms have the mass number of 35. It agrees with the value from the table above.
Summary
- The atomic mass of an element is the weighted average of the atomic masses of the naturally occurring isotopes of that element.
- Calculations of atomic mass use the percent abundance of each isotope.
Contributors and Attributions
Chlorine Atomic Mass And Number
CK-12 Foundation by Sharon Bewick, Richard Parsons, Therese Forsythe, Shonna Robinson, and Jean Dupon.
